AW and AP equally contributed to the study
Introduction: There is no standard salvage therapy in relapsed/refractory AML (R/R AML). CPX-351, a dual-drug liposomal encapsulation of daunorubicin (DNR) and cytarabine (AraC) in a synergistic 1:5 molar ratio, shows effective antileukemia activity ( Lancet JE, JCO 2018). Favorable results of cladribine-based salvage regimens (CLAG-M, CLAG) ( Wierzbowska A; Eur J Hematol 2008, Scheckel CJ, Leuk Res. 2020) as well as first-line therapies ( Hołowiecki J, JCO 2012; Kadia TM Lancet Haematol 2021, Kadia TM JCO 2022) confirmed a value of cladribine in the treatment of AML.
Aim: This phase I study was designed to explore safety (MTD), toxicity and efficacy of increasing doses of cladribine in combination with standard dose of CPX-351 in R/R AML patients (pts). (EudraCT number: 2020-002535-29).
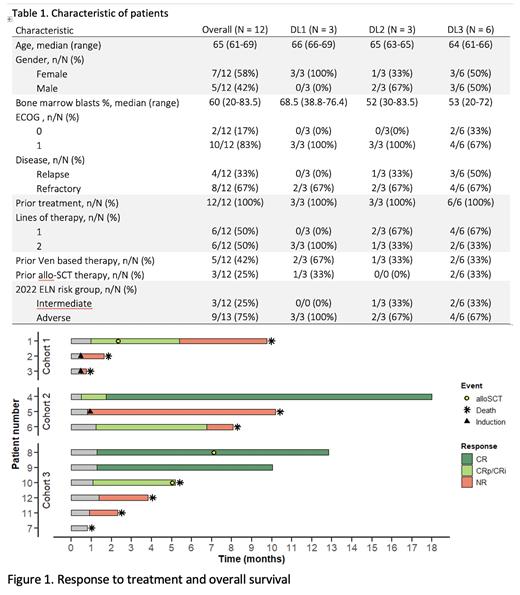
Results: Twelve pts were enrolled with a median age 65 (range 61-69) years. Importantly, 8 pts (67%) were refractory to the former first- or second- line treatment. Nine pts (75%) were in the adverse risk group according to ELN 2022 including 4 pts with TP53 mut. Four pts (33%) were refractory to venetoclax (VEN)-based therapy and 3 pts (25%) had previous alloHCT (Table 1). Three patients (2 in DL1 and 1 in DL2) required two inductions. One patient in DL3 experienced a CNS and GI hemorrhage due to thrombocytopenia refractory to platelet transfusion, which was considered a DLT requiring enrollment of additional 3 pts. No patients discontinued therapy due to intolerance.
Toxicities of grade ≥3 during the induction cycle were mainly infections. All patients developed infections during 1-st cycle of treatment. Blood stream infections (n=5; 42%), febrile neutropenia (n=4; 33%), and pneumonia (n=3; 25%) were the most common infectious events. One episode of grade 5 hemorrhage occurred in a patient with thrombocytopenia refractory to platelet transfusions. Cytopenias in responding pts treated with CPX-351 and cladribine were similar to that observed with CPX-351 alone.
The composite CR (cCR=CR+CRi+CRp) rate was 50% (6/12 pts), 5 pts (42%) not responded and 1 patient died due to CNS hemorrhage before response evaluation but with significant BM blasts clearance at D14. cCR was achieved in 2 out of 3 pts (66%) with intermediate-risk and 4/9 pts (44%) with adverse-risk according ELN 2022. Within the high-risk population, pts with TP53 mut had lower probability to achieve remission (1/4; 25%) than pts with myelodysplasia related mutations (3/5; 60%). No remissions were observed in pts refractory or relapsed after VEN-based therapy.
Measurable residual disease (MRD)-negative cCR assessed via multiparameter flow cytometry was achieved in 60% ( n = 3/5) of MRD-evaluable pts, including all pts (n = 3/3; 100%) in DL3.
All pts achieving cCR had post remission therapy with one (n=1; 8%) or two (n=5; 92%) courses of CPX-351+ cladribine consolidation and 3 pts proceeded to alloHCT. After a median (Me) follow-up of 12.8 months (mos), Me overall survival (OS) was 6.6 mos (95% CI, 2.3-not reached [NR]) for all pts and 9.8 mos (95% CI, 8.07-NR) for those who achieved cCR. Median event-free survival (EFS) was 3.28 mos (95% CI, 0.8-NR) for the study population.
Conclusions: Combination of cladribine with standard dose of CPX-351 is well tolerated. Cladribine dose 5 mg/m 2 was selected as RP2D. Safety profile does not indicate any significant additional myelosuppression. Cladribine with CPX-351 shows satisfying clinical activity with cCR rate of 50%, cCR-MRD neg of 60% and durable remission in some R/R AML patients. Further evaluation of the efficacy and safety of this regimen is needed.
OffLabel Disclosure:
Wierzbowska:Gilead: Honoraria; Pfizer: Honoraria; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria; Novartis: Honoraria; Astellas: Consultancy, Honoraria; Servier: Honoraria; JazzPharmaceuticals/swixx: Honoraria. Pluta:Jazz Pharmaceuticals (Swixx): Honoraria, Research Funding; Celgene/BMS: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Abbvie: Honoraria. Stelmach:Novartis: Honoraria. Czemerska:Celgene/BMS: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Sandoz: Honoraria. Sobas:Abbvie: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria. Rybka:Pfizer: Honoraria; Sanofi: Honoraria; Roche: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Celgen/BMS: Honoraria; Abbvie: Honoraria; Novartis: Honoraria. Wróbel:Abbvie: Honoraria; Gilead: Honoraria; Celgen/BMS: Honoraria; Roche: Honoraria, Research Funding; Novartis: Honoraria; Janssen: Honoraria; Amgen: Honoraria, Research Funding; Takeda: Honoraria; Beigene: Honoraria; GSK: Honoraria. Prejzner:Novartis: Honoraria; BMS: Honoraria; Pfizer: Honoraria. Zaucha:Takeda: Honoraria; BMS: Research Funding; Roche: Honoraria; Janssen: Honoraria; MSD: Research Funding; Novartis: Honoraria; Gilead: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gil:Abbvie: Honoraria; Astellas: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; Janssen: Honoraria. Giebel:Zentiva: Consultancy, Honoraria; BMS: Honoraria, Speakers Bureau; Angelini: Honoraria, Speakers Bureau; Swixx: Honoraria, Speakers Bureau; Servier: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau.
Cladribine in combination with CPX-351 in AML

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal